Irb Continuing Review of an Active Trial is Commonly Conducted Once a Year
Return to Education Homepage
According to federal regulations, the IRB must conduct Continuing Review of previously approved research at intervals appropriate to the degree of risk, but not less than once per year [45 CFR 46.109(e) (DHHS) and 21 CFR 56.109(f) (FDA)]. The revised Common Rule (2018 Requirements) outlines circumstances where Continuing Review is not required unless otherwise determined by the IRB, and in these situations, PSU will conduct an annual Administrative Review. If your study requires Continuing or Administrative Review, this will be outlined in the study approval letter and the PI will receive reminders from CATS IRB about submitting the appropriate review type. These reviews must be submitted to and reviewed by the IRB prior to the approval end date or Administrative Review due date for the study.
Failure to submit the required form before the deadline moves the study to a lapsed state and requires all research activities to cease until the Continuing or Administrative Review is complete. If a study is in a lapsed state for 45 days, the IRB office will automatically close the study administratively and the study cannot be re-activated at that time.
Researchers are strongly encouraged to submit Continuing and Administrative Review several weeks prior to the deadline.
The image below depicts the main study workspace in CATS IRB indicating that an Administrative Review is required. This main study workspace is also where Continuing Review will be noted.
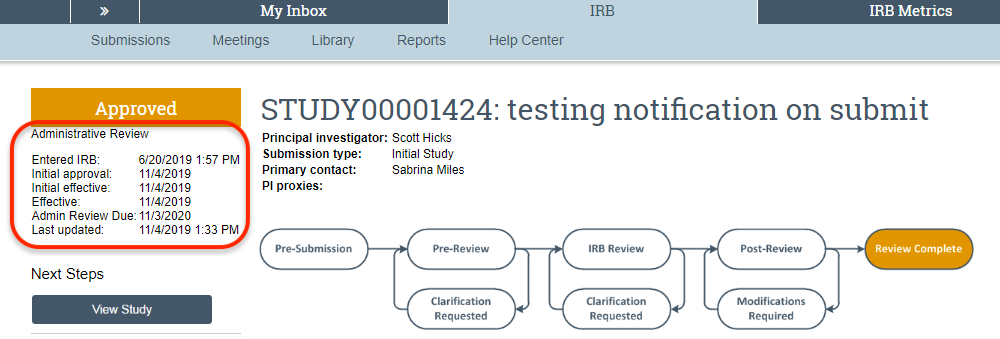
Continuing Review
Continuing Reviews are submitted to close a study or extend the approval period. Depending on the type of research and research milestones that have been completed, Continuing Review may be performed by expedited review or by full committee review. Continuing Review is not required for studies that have been granted an exemption or not human subjects research determination.
Administrative Review
When Continuing Review is not required by the IRB, an annual Administrative Review by the IRB program will be required. Even when a Continuing Review is not required, investigators are still responsible for submitting modifications prior to implementation and new information to the IRB. The IRB requires less information for Administrative Reviews than Continuing Reviews. For example, enrollment totals are not required for an Administrative Review.
Submitting a Continuing or Administrative Review
Continuing and Administrative Reviews follow the same submission process in CATS IRB. Follow the steps below outlined below to begin a Continuing or Administrative Review:
- Login to CATS IRB using your PSU access account.
- Click "IRB" from the top tab menu.
- Select the desired study be clicking on the "Active study" tab and clicking on the study name.
- Click "Create Modification/CR" on the left side of the screen and then select "Continuing Review." (See image below.)


Note: If any changes are being made, select "Modification and Continuing Review" and refer to the instructions on how to Submit a Modification.
- Complete all questions applicable to Continuing Review/Administrative Review.
- Continuing Review Only:Upload any pertinent documents as outlined in question #4 in the Continuing Review form.
- Click "Save and Exit" to return to the Submission workspace where you MUST SELECT "Submit" to send to the IRB for review.
 Only the PI or the PI Proxy can Submit. If Submit is not selected, the continuing review will remain in the pre-submission state and will not be submitted.
Only the PI or the PI Proxy can Submit. If Submit is not selected, the continuing review will remain in the pre-submission state and will not be submitted.
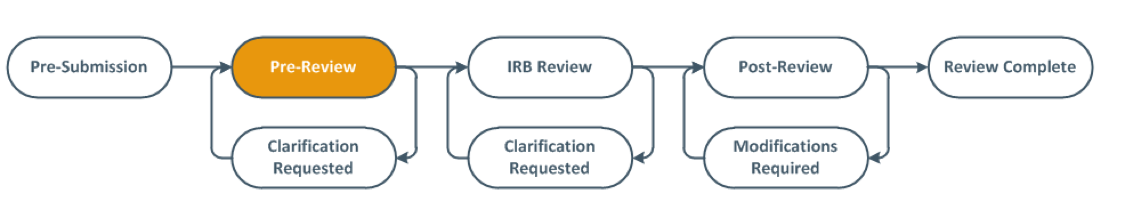
- Once the Continuing or Administrative Review is submitted, the state will move to "Pre-Review" as pictured below. The PI, PI Proxy, and the Primary Contact will be notified by email when the Continuing Review is approved or the Administrative Review is acknowledged.
Source: https://www.research.psu.edu/irb-reviews
Enviar um comentário for "Irb Continuing Review of an Active Trial is Commonly Conducted Once a Year"